how is a covalent bond formed
An atom that shares one or more of its electrons will. A covalent bond is formed between two atoms by sharing electrons.

How Is Covalent Bond Is Formed A Plus Topper Formationofoxygenmolecule Covalent Bonding Bond Hydrogen Atom
A chlorine atom has seven valence electrons.

. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions. Atoms can fill up their outer electron shell and achieve stability by exchanging their outermost valence. Share a pair of electrons. Forming a covalent bond A covalent bond is formed when two atoms share a pair of electrons.
The electrons involved are in the outer shells of the atoms. As a result the shared electrons will be closer. An ionic bond is a type of chemical bond in which the atoms have different electronegativity values from each other. Bonds form if there is an overlap of two atomic orbitals.
The pair of electrons participating in this type of bonding is called shared pair or bonding pair. The bonded pair is the glue that holds the atoms together. In order to achieve further stability which is gained by forming a complete electron shell atoms can covalently bond with other atoms. Covalent bond is formed by sharing of valence electrons.
The distance between two bonded atoms at their minimum potential energy. The energy required to break the bonds in one mole of a chemical compound. The covalent bonds are also termed as molecular bonds. When one electron pair is shared it is single covalent bond when two electron pairs are shared it is double covalent bond and when three electron pairs are shared it is triple covalent bond.
Covalent bonding occurs when the electronegativity difference between elements atoms is zero or relatively small. By sharing their outer most valence electrons atoms can fill up their outer electron shell and gain stability. Hydrogen bond is a weak electrostatic attraction between the hydrogen and an electronegative atom due to their difference in electronegativity. Shared electrons located in the space between the two nuclei are called bonding electrons.
How is dative bond formed. A dative bond is a covalent bond between two atoms where one of the atoms provides both electrons that form the bond. As in NH3 three valence electrons of N are being shared with three hydrogen atoms so we obtain total three electron pairs being shared between N and H in NH3 so. H forms only one bond because it.
Polar Covalent Bond. Covalent compounds are formed by the mutual sharing of electrons by the combining atoms. The H2 molecule is stable because each hydrogen atom now has a shared pair of electrons and has achieved a stable noble gas configuration. Covalent bonding occurs in most non-metal elements and in compounds formed between non-metals.
Sharing of bonding pairs will ensure that the atoms achieve stability in their outer shell which is similar to the atoms of noble gases. But the question is why does sharing a pair of electrons make the atoms stick together is a covalent bond. These shared electrons are called valence electrons. The covalent bonds in a polyatomic ion can be represented using the Lewis formulation.
The atom with the higher electronegativity will have a stronger pull for electrons Similiar to a Tug-O-War game whoever is stronger usually wins. A bond formed when atoms share one or more pairs of electrons. A Polar Covalent Bond is created when the shared electrons between atoms are not equally shared. Two atoms that are covalently bonded have less energy than the individual atoms making the bonded atoms more stable.
Covalent bonds form when atoms share their valence electrons with other atoms to become a more stable molecule. This occurs when one atom has a higher electronegativity than the atom it is sharing with. Covalent bonds are strong bonds with greater bond energy. Covalent bond is formed by sharing electrons between non-metals Explanation.
Atoms share their electrons in order to completely fill up their outer-most layer the valence shell. Covalent bonding is a type of bonding formed only between non-metal elements with some exceptions. Covalent bond is formed when there is sharing of electron pairs. Oxygen has 6 electrons in valence shell.
This is due to electronegativity difference between the elements is less than 17. However in a covalent bond the atoms are bound to share electrons. A covalent bond forms when two or more valence electrons are attracted by the positively charged nuclei of two atoms and thus are shared between both atoms. Here Hydrogen has 1 electron in valence shell it needs one to complete its duplet.
For example if we talk about water H2O it is a polar covalent bond. How are Covalent Bonds Formed. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. A covalent bond forms when two non-metal atoms.
Formation of some typical covalent compound is described below. In a typical covalent bond each atom supplies an electron to form the bond. Ions that have opposite charges will form an ionic bond in such a way that total charge on the compound is 0. The electron pair is attracted to both atomic nuclei holding them together to form a bond.
How is a Covalent Bond Formed. Covalent bonding occurs when pairs of electrons are shared by atoms. This bonding is formed by sharing a pair of an electron to achieve a full outmost shell. Bond formed between a metal and non metal atom is usually a lonie bond b covalent bond c coordinate bond How are covalent bonds formed.
A covalent bond is formed when two atoms share one or more pairs of electrons. A covalent bond is formed by equal sharing of electrons from both the participating atoms. Covalent bond is a primary chemical bond formed by the sharing of electron pairs. Formation of a chlorine molecule.
As pairs of electrons are exchanged between atoms covalent bonding happens. A covalent bond occurs when two non-metal atoms share their electrons to achieve the noble gas electron configuration. Hydrogen is an exception to the octet rule. For example sodium Na and chlorine Cl form an ionic bond to make NaCl table salt.
Thus chlorine atom requires only one electron to acquire a. Atoms will covalently bond with other atoms in order to gain more stability which is gained by forming a full electron shell. The electronic configuration of chlorine 2 8 7. Instead of giving or receiving electrons each atom will share electrons by overlapping their outer most orbit.

Ionic And Covalent Bonding Are Depicted In The Picture Ionic Bonds Is The Attraction Of A Cation To An An Ionic Bonding Teaching Chemistry Covalent Bonding
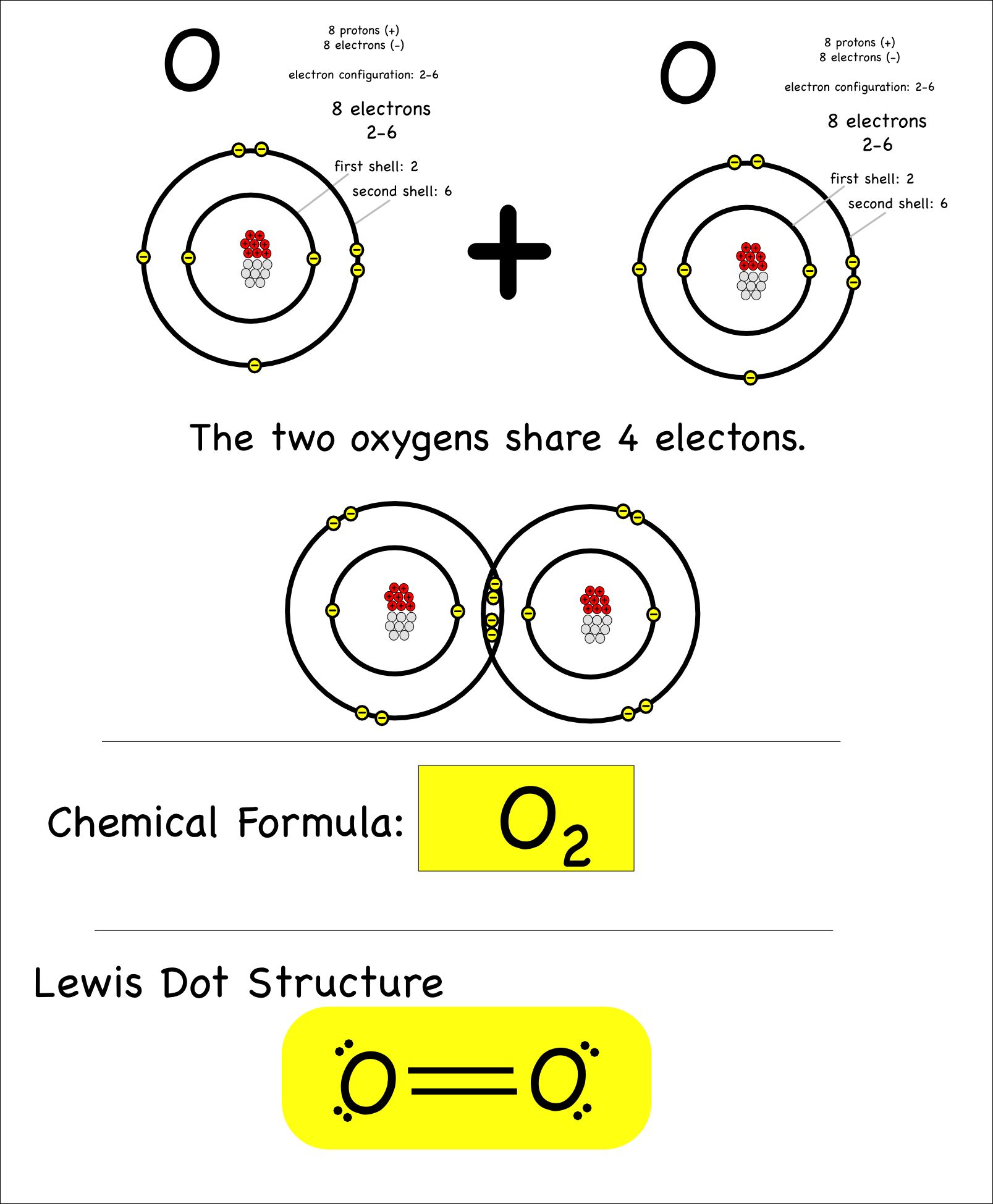
Covalent Bonding In An Oxygen Molecule Covalent Bonding Chemistry Worksheets Chemistry Classroom

How Is Covalent Bond Is Formed A Plus Topper Formationofoxygenmolecule Covalent Bonding Bond Form Example

How Is Covalent Bond Is Formed A Plus Topper Formationofcovalentbond Covalent Bonding Bond Form Example

Covalent Bond Formed Example 13 Covalent Bonding Bond Form Example

How Is Covalent Bond Is Formed A Plus Topper Formationofcovalentbond In 2021 Covalent Bonding Chemistry For Kids Bond
Komentar
Posting Komentar